Tests & Types
| Main Test | Purpose |
| hemogram | The hemogram test, also known as HMG or Complete Hemogram Test, is a set of tests including complete blood count (CBC) and erythrocyte sedimentation rate (ESR) that help to assess the body’s overall health and aids in the detection of a variety of illnesses, including anaemia, infection, and leukaemia. |
| Apolipoprotein A | This test measures the amount of apolipoprotein A in your blood. It helps your healthcare provider figure out your risk for cardiovascular disease. Apolipoprotein A is a protein carried in HDL (“good”) cholesterol. It helps start the process for HDL to remove bad types of cholesterol from your body. |
| Apolipoprotein B | An Apo B or Apolipoprotein B-100 test is a blood test that can tell you about your risk for cardiovascular (heart and blood vessel) disease. To do this, it measures the amount of Apo B, which carries substances in your blood that help make plaque, a waxy fat that can block your arteries |
| HIGH SENSITIVITY C-REACTIVE PROTEIN (HS-CRP) | Disclaimer: Persistent unexplained elevation of HSCRP >10 should be evaluated for non-cardiovascular etiologies such as infection , active arthritis or concurrent illness. High sensitivity C- reactive Protein ( HSCRP) can be used as an independent risk marker for the identification of Individuals at risk for future cardiovascular Disease. A coronary artery disease risk assessment should be based on the average of two hs-CRP tests, ideally taken two weeks apart. |
| Lipid | A cholesterol (or lipid profile) blood test looks at the levels of cholesterol and other fats in your blood. You might need this test |
HEMOGRAM
RED CELL DISTRIBUTION WIDTH – SD(RDW-SD) – LOW :
HIGH SENSITIVITY C-REACTIVE PROTEIN (HS-CRP)
High-sensitivity C-reactive protein (hsCRP) is a marker of inflammation that predicts incident myocardial infarction, stroke, peripheral arterial disease, and sudden cardiac death among healthy individuals with no history of cardiovascular disease, and recurrent events and death in patients with acute or stable coronary syndromes
What is the most common cause of high CRP?
Lipid Test : https://www.youtube.com/watch?v=9dghtf7Z7fw
Overview
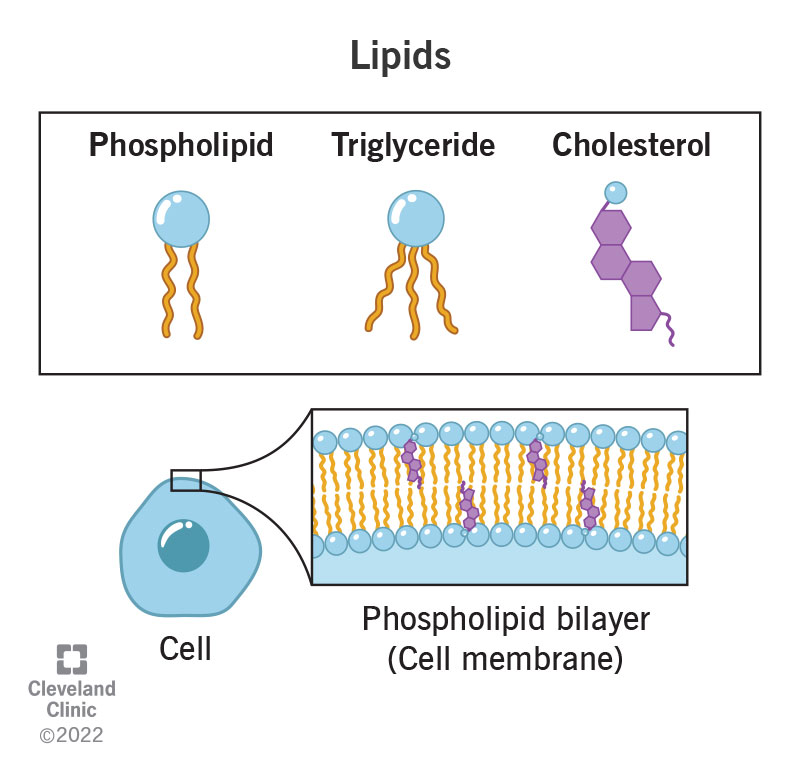
What are lipids?
Lipids are chemical compounds (elements that make a chemical bond) in your body that help with some of its functions. These are fatty or waxy substances your body makes that don’t dissolve in water.
Examples of lipids
Cholesterol is a lipid in your blood. Your body needs it to help you take in fats and vitamins and make hormones. Cholesterol and triglycerides avoid water, so they can’t travel through blood themselves. This is why they combine with proteins to make lipoproteins that can move throughout your body.
You’ll recognize some lipids by their nicknames: HDL (high-density lipoproteins) and LDL (low-density lipoproteins).
Your liver and small intestine make HDL, which carries cholesterol into lipoproteins or your liver. Your liver converts it to bile acid so you can get rid of it. HDL (the “good” cholesterol) also fights inflammation, blood clots and oxidation.
Your liver makes VLDL (very low-density lipoproteins), which takes triglyceride from your liver to other cells. When VLDLs drop off triglycerides and cholesterol, they get denser and become LDL or the “bad” cholesterol. LDLs can stick to your artery walls and make it harder for blood to get through your arteries.
What are lipids made of?
Oxygen, carbon and hydrogen bond to each other to form lipids. Lipids like cholesterol are part of your cell membranes. They give your cells structure and allow substances to go in and out of your cells.
Cholesterol is important:
Cholesterol is a fat-like, waxy substance that helps your body make cell membranes, make hormones like estrogen, testosterone and adrenal hormones. help your metabolism work efficiently. cholesterol is essential for your body to produce vitamin D. The cholesterol in your blood comes from two sources: the foods you eat and your liver. Your liver makes all the cholesterol your body needs.
Its main function is to maintain the integrity and fluidity of cell membranes and to serve as a precursor for the synthesis of substances that are vital for the organism including steroid hormones, bile acids,
References: https://www.youtube.com/watch?v=9dghtf7Z7fw
Uric Acid:
Uric acid is synthesized mainly in the liver, intestines and the vascular endothelium as the end product of an exogenous pool of purines, and endogenously from damaged, dying and dead cells, whereby nucleic acids, adenine and guanine, are degraded into uric acid.
Uric acid is a chemical created when the body breaks down substances called purines. Purines are normally produced in the body and are also found in some foods and drinks. Foods with high content of purines include liver, anchovies, mackerel, dried beans and peas, and beer.
Purines are natural substances found in all of the body’s cells and in virtually all foods. In humans, purines are metabolized to uric acid, which serves as an antioxidant and helps to prevent damage caused by active oxygen species. A continuous supply of uric acid is important for protecting human blood vessels. However, frequent and high intake of purine-rich foods reportedly enhances serum uric acid levels, which results in gout and could be a risk factor for cardiovascular disease, kidney disease, and metabolic syndrome.
A high protein diet can create high uric acid levels as purines in the protein (not in eggs and non fermented dairy) convert to uric acid which can result in gout.
Too much exercise in the alteration of uric acid levels. (A) Cellular component-strenuous exercise causes an in- creased turnover of tissue ATP( adenosine triphosphate) leading to an increase in the purine pool, the immediate precursor of uric acid.
ATP:
It is often referred to as the energy currency of the cell and can be compared to storing money in a bank. ATP can be used to store energy for future reactions or be withdrawn to pay for reactions when energy is required by the cell. Animals store the energy obtained from the breakdown of food as ATP.
Causes of a high uric acid level in the blood include: Diuretics (water retention relievers) Drinking too much alcohol. Drinking too much soda or eating too much of foods that contain fructose, a type of sugar.
Purines and Pyrimidines
Purines and pyrimidines are the two families of nitrogenous bases that make up nucleic acids – in other words, they are the building blocks of DNA and RNA
What are Purines and Pyrimidines?: The Basics
Each DNA strand has a ‘backbone’ that is made up of a sugar-phosphate chain. Attached to each one of these sugars is a nitrogenous base that is composed of carbon and nitrogen rings. The number of rings this base has determines whether the base is a purine (two rings) or a pyrimidine (one ring). The purines on one strand of DNA form hydrogen bonds with the corresponding pyrimidines on the opposite strand of DNA, and vice versa, to hold the two strands together. Within DNA molecules, this is their most important function and is known as base pairing. Because hydrogen bonds are not as strong as covalent bonds, base pairings can easily be separated, allowing for replication and transcription.
Because purines always bind with pyrimidines – known as complementary pairing – the ratio of the two will always be constant within a DNA molecule. In other words, one strand of DNA will always be an exact complement of the other as far as purines and pyrimidines go.This phenomenon is known as Chargaff’s Rule, named after Irwin Chargaff, who first noticed it. This complementary pairing occurs because the respective sizes of the bases and because of the kinds of hydrogen bonds that are possible between them (they pair more favorably with bases with which they can have the maximum amount of hydrogen bonds).
Types of Purines and Pyrimidines
There are two main types of purine: Adenine and Guanine. Both of these occur in both DNA and RNA. There are three main types of pyrimidines, however only one of them exists in both DNA and RNA: Cytosine. The other two are Uracil, which is RNA-exclusive, and Thymine, which is DNA-exclusive. One strategy that may help you remember this is to think of pyrimidines like pyramids that have sharp and pointy tops. So sharp and pointy in fact, that they might CUT (Cytosine, Uracil, Thymine) you.
Which purines pair with which pyrimidines is always constant, as is the number of hydrogen bonds between them:
- ADENINE pairs with THYMINE (A::T) with two hydrogen bonds
- GUANINE pairs with CYTOSINE (G::C) with three hydrogen bonds
One way to remember which bases go together is to look at the shapes of the letters themselves. The letters made up of only straight lines (A and T) are paired with each other, while the letters that are made up of curves (G and C) also go together. Just make sure you don’t write your A’s in cursive!
Purines vs. Pyrimidines
When it comes to identifying the main differences between purines and pyrimidines, what you’ll want to remember is the ‘three S’s’: Structure, Size, and Source. The very basics of what you need to know are in the table below, but you can find more details about each one further down.
Purines |
Pyrimidines |
|
Structure |
Double carbon-nitrogen ring with four nitrogen atoms | Single carbon-nitrogen ring with two nitrogen atoms |
Size |
Bigger | Smaller |
Source |
Adenine and Guanine in both DNA and RNA | Cytosine in both DNA and RNA Uracil only in RNA Thymine only in DNA |
