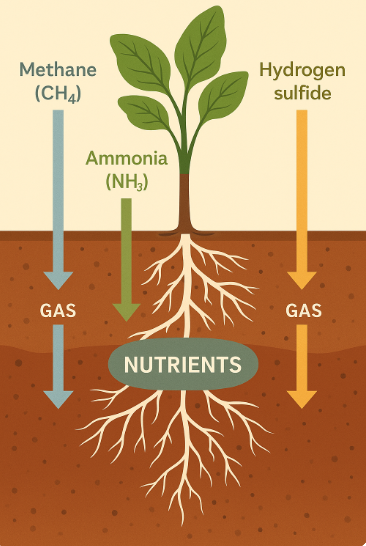
Excellent scientific question! Let’s explore how the three gases produced during Jeevamrutham fermentation — methane (CH₄), ammonia (NH₃), and hydrogen sulfide (H₂S) — interact with plants and soil:
🧪 These gases are byproducts of microbial activity, not directly made for plants — but they do have indirect roles:
1. 🌫️ Methane (CH₄)
Aspect |
Details |
|---|---|
Source |
Anaerobic microbes break down organic matter without oxygen. |
Role in plants |
❌ Plants do not use methane directly. |
Soil impact |
In low-oxygen soils, excess methane indicates unhealthy microbial balance. |
Risk |
Too much methane = anaerobic, suffocating soil, weak root growth. |
Indirect benefit |
Small amounts show active decomposition; supports methane-consuming bacteria, which balance soil gases. |
2. 💨 Ammonia (NH₃)
Aspect |
Details |
|---|---|
Source |
From urea/cow urine breakdown, protein decomposition by bacteria. |
Role in plants |
✅ Ammonia converts to ammonium (NH₄⁺) in moist soil — which plants can absorb as nitrogen. |
Soil impact |
Helps build green leafy growth (like spinach, coriander, etc.). |
Risk |
If ammonia is too high, it can burn roots or turn soil alkaline. |
Tip |
Moist soil + balanced microbes = ammonia safely converted into usable plant food. |
3. 💀 Hydrogen Sulfide (H₂S)
Aspect |
Details |
|---|---|
Source |
Anaerobic bacteria break down sulfur in organic matter. |
Role in plants |
❌ Toxic to roots in high amounts. |
Soil impact |
Even low levels harm root respiration. Causes rotten egg smell. |
Risk |
Indicates oxygen starvation in soil. |
Good sign? |
🚫 No — H₂S is a sign to reduce watering or improve soil aeration. |
🧪 Summary Table: Gas Behavior in Soil
Gas |
Useful to Plant? |
When Beneficial |
When Harmful |
|---|---|---|---|
Methane (CH₄) |
❌ No |
In tiny amounts, shows microbial activity |
Excess = anaerobic, poor roots |
Ammonia (NH₃) |
✅ Yes (converted to NH₄⁺) |
Provides nitrogen |
In excess = root burn, smell, pests |
Hydrogen sulfide (H₂S) |
❌ No |
None |
Damages roots, smells bad, anaerobic zone |
💡 How to Handle These Gases in Jeevamrutham Use:
Tip |
Benefit |
|---|---|
Always apply on moist but not wet soil |
Prevents anaerobic pockets and root damage |
Stir and ferment Jeevamrutham only 2–3 days |
Prevents overproduction of gases |
Use in small doses (5–10 L/bed) |
Gives microbial benefit without gas overload |
Loosen soil if smell is strong after application |
Releases trapped gases, saves roots |
How Ammonia converts into NH4+:
Great question! Here’s a technical explanation of how ammonia (NH₃) gets converted into forms that plants can absorb — mainly ammonium (NH₄⁺) — in the soil.
🧪 Step-by-Step: How Ammonia Works in Soil
🟢 1. Ammonia Formation (NH₃)
- Ammonia is released into the soil from:
- Cow urine (urea → NH₃ via urease enzyme)
- Organic matter decomposition
- Jeevamrutham fermentation
🔁 2. Ammonia to Ammonium Conversion
- In the presence of moisture and slightly acidic soil, ammonia gas (NH₃) dissolves into water and forms:
NH₃ (ammonia)+H⁺ (from water)→NH₄⁺ (ammonium ion)\text{NH₃ (ammonia)} + \text{H⁺ (from water)} → \text{NH₄⁺ (ammonium ion)}
✅ NH₄⁺ = Plant-usable form
- This ammonium ion is stable, non-volatile, and directly absorbed by plant roots.
- Especially useful for leafy vegetables which need quick nitrogen for green growth.
🔁 BONUS: What Happens Next (Optional Advanced)
| Process | Reaction | Notes |
|---|---|---|
| Nitrification | NH₄⁺ → NO₂⁻ → NO₃⁻ | Microbial conversion to nitrate (NO₃⁻), another plant-usable form |
| Immobilization | NH₄⁺ → taken by microbes | Happens when too much carbon (e.g. dry mulch) is present |
| Volatilization | NH₃ gas escapes to air | Happens if soil is dry, hot, or alkaline (waste of nitrogen) |
📌 Key Factors That Help Ammonia Work for Plants:
| Factor | Ideal Range | Why it Matters |
|---|---|---|
| Soil pH | 6.0–7.5 | Keeps NH₄⁺ stable and prevents gas loss |
| Moisture | 50–70% field capacity | Helps dissolve NH₃ into NH₄⁺ |
| Temperature | 20–30°C | Supports microbial nitrogen cycling |
| Aeration | Medium | Encourages nitrifying bacteria without drying out soil |
🧠 Summary:
- Ammonia (NH₃) is not directly taken by plants.
- It must first react with water and H⁺ ions to form ammonium (NH₄⁺).
- NH₄⁺ is safe, stable, and readily absorbed by plant roots.
Would you like a simple flowchart or visual to show this ammonia-to-ammonium-to-nitrate conversion cycle?
